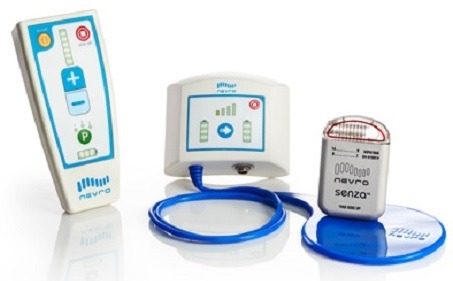
The Therapeutic Goods Administration (TGA) has granted approval to Nevro for its next-generation Senza II spinal cord stimulation system delivering the company’s proprietary HF10 therapy. According to Nevro, the Senza II system offers the outcomes and clinical advantages of HF10 therapy through a smaller and more refined footprint, while maintaining the performance, durability, and full-body magnetic resonance imaging conditional labelling of their current implantable pulse generator.
“My colleagues and I are excited about the approval of the Senza II spinal cord stimulation system,” says Paul Verrills, director of the Metro Pain Group, Melbourne, Australia. “The reduced size and optimised design of the Senza II implantable pulse generator allow for greater patient comfort and placement options. Most importantly, it delivers HF10 therapy providing profound and paraesthesia-free pain relief for patients.”
In addition to this most recent TGA approval, the Senza II spinal cord stimulation system also has FDA approval and CE mark clearance.
HF10 therapy is a spinal cord stimulation therapy that provides electrical pulses to the spinal cord to alleviate pain. The electrical pulses are delivered by small electrodes on leads that are placed near the spinal cord and are connected to a compact, battery-powered generator implanted under the skin. HF10 therapy is the only spinal cord stimulation therapy indicated to provide pain relief without paraesthesia, and is also the first spinal cord stimulation therapy to demonstrate superiority to traditional spinal cord stimulation for back and leg pain in a comparative pivotal study.









