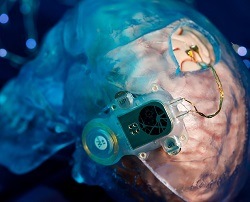
The Wyss Center for Bio and Neuroengineering in Geneva, Switzerland has today revealed the latest preclinical neural data acquired with its fully implantable ABILITY brain-computer interface (BCI) system. The results, to be presented at the Society for Neuroscience meeting (12–16 November 2022, San Diego, USA), are accompanied by new details of the ABILITY system demonstrating its flexibility in connecting to different electrode types.
ABILITY, a wireless implantable medical device, is being developed by the Wyss Center team together with academic and clinical collaborators, and a network of industrial technology partners.
Intended for long-term implantation and home use, ABILITY is designed to enable applications like restoration of communication and movement for people with severe paralysis resulting from amyotrophic lateral sclerosis (ALS), brainstem stroke or spinal cord injury.
The preclinical feasibility studies recorded brain activity in sheep with intracortical microelectrode arrays (MEAs) and electrocorticography grid (ECoG) electrodes implanted beneath the skull, a Wyss Center press release details.
“These new results are an important step towards demonstrating safety and efficacy of the device while recording and transmitting neural data in real time over a period of months,” said Shenandoah Montamat (Wyss Center, Geneva, Switzerland), who is managing the ABILITY project. “The device performance and quality of the data are very encouraging as we prepare next steps towards human clinical trials.”
Two MEAs, each smaller than a pea, recorded the activity of individual neurons with an array of fine needles that penetrated the surface of the cortex. At this resolution, it is possible to decode fine movement intention with high accuracy from very small brain areas. In a separate study, four ECoG grids, each about the size of a postage stamp, recorded signals from the surface of the cortex. ECoG electrodes cover a larger area of the brain than MEAs and measure the combined activity of nearby neurons with sufficient resolution to decode speech in humans.
The ABILITY system records 128 channels of neural data at a frequency sufficiently high to observe communication between single neurons, known as action potentials, as well as the lower frequency synchronous activity of groups of neurons firing together, known as local field potentials, the release continues. It wirelessly transmits the raw data through the skin using a high-speed optical link. Wearable components send the neural data to a computer with a wired connection. The wearable also wirelessly powers the implant through the skin via induction.
“The development of ABILITY draws on experience from a recent clinical case study that successfully enabled BCI communication for a person completely locked-in because of ALS,” added Jonas Zimmermann, senior neuroscientist at the Wyss Center.
In that study, the patient learned to modulate neural activity to control speller software. The system used a wired connection to transfer data between implanted electrodes and an external computer through a percutaneous connection in the scalp.
“The study highlights the clinical need for breakthrough implant technology to improve ease of use for patients and caregivers,” noted Zimmermann.
George Kouvas, Wyss Center chief technology officer, said: “At the Wyss Center we are addressing some of the major neuroscientific and engineering challenges that fully implantable BCIs encounter during development. The result is a versatile technology like ABILITY that has the potential to help the BCI market grow.”
Human clinical trials are now being prepared to assess ABILITY system performance and understand the acceptance of implantable BCIs by patients, caregivers, and healthcare professionals, the release concludes.









