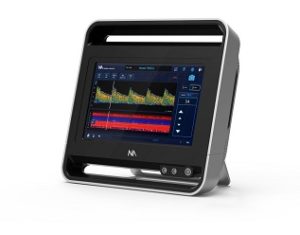
Neural Analytics has announced the commercial launch of its Lucid M1 Transcranial Doppler Ultrasound System, a portable brain monitoring system designed to measure cerebral blood flow velocities within the head and neck.
The system was launched at the Society of Vascular and Interventional Neurology annual meeting (SVIN; 16–19 November, Brooklyn, USA).
According to the company, the Lucid System uses a type of ultrasound called Trans Cranial Doppler to assess the brain’s blood vessels from outside the body. This analysis is non-invasive, can be performed in the physician’s office, and can help the physician diagnose brain disorders, potentially without the need for additional, more invasive tests. Many significant brain disorders, such as severe traumatic brain injury (TBI), are caused by blood flow disruption.
The Lucid System is a battery operated medical grade tablet device designed to be moved easily throughout a medical facility in a range of settings that require the rapid assessment of blood flow in the brain to expedite treatment.
The Lucid System received US FDA clearance recently and is the first FDA cleared fully portable all-in-one ultrasound system designed for rapid triaging and monitoring of patients with brain disorders. The system is the first product that Neural Analytics will sell commercially.









