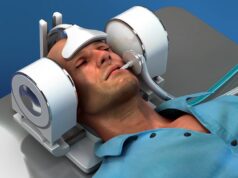
MindRhythm has announced that it has officially submitted a de novo application to the US Food and Drug Administration (FDA) seeking clearance for its Harmony device designed for prehospital large vessel occlusion (LVO) stroke identification.
The company’s novel, non-invasive Harmony headset device—embedded with HeadPulse biometric analysis technology—was developed to address the limitations of prehospital stroke identification in “a simple and efficient way”.
Using Harmony’s rapid ‘LVO yes/no’ information, emergency medical services (EMS) professionals can confidently triage LVO patients to thrombectomy centres and send non-LVO patients to the closest stroke centre, expediting access to critical treatment for all stroke patients, according to a MindRhythm press release.
“This is a significant milestone for MindRhythm and stroke care,” commented MindRhythm chief executive officer (CEO) John Keane. “The addition of Harmony’s objective biometric data to a clinical assessment allows EMS professionals to more effectively triage stroke patients with the goal of expediting time to treatment. MindRhythm’s ultimate objective is to improve outcomes in this life-threatening condition.”
This de novo submission follows two previous milestones achieved by MindRhythm: Harmony’s Breakthrough Device designation in 2023 and successful completion of EPISODE—one of the largest prehospital studies of a stroke identification device ever conducted, according to the company.
Based on these accomplishments and EMS feedback, MindRhythm says it is anticipating broad adoption when Harmony is available following US FDA clearance.