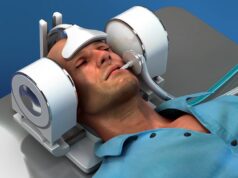
MindRhythm has announced that the US Food and Drug Administration (FDA) has awarded a Breakthrough Device designation to its Harmony headset embedded with HeadPulse technology.
“This designation highlights the position that Harmony is truly breakthrough, as it acknowledges the importance of this product for our patients and the likelihood that MindRhythm’s pivotal trial will be successful,” said Wade Smith (University of California San Francisco [UCSF], San Francisco, USA), co-founder and chair of the scientific advisory board at MindRhythm. “We appreciate the high standards the FDA requires as it adds confidence to medical professionals that the use of the device is in the best interests of our stroke patients.”
The function of MindRhythm’s Harmony device is to aid in the assessment of suspected stroke patients in the prehospital setting to determine whether the patient may have a large vessel occlusion (LVO) stroke.
MindRhythm chose to pursue breakthrough designation in advance of its submission for FDA clearance with the belief that Harmony meets all primary and secondary criteria for the programme, including the fact that device availability is in the best interest of patients.
According to a press release from the company, there is currently no device available that can easily and accurately identify LVO before a patient arrives at a hospital.
Harmony is designed to help emergency medical services (EMS) professionals determine the presence or absence of LVO stroke. This information can be used by EMS to transport patients to the closest hospital that can deliver the most appropriate level of skilled care for their stroke type.
“Time to treatment is absolutely critical for favourable outcomes in all strokes,” said MindRhythm CEO John Keane. “Excellent treatments are available for stroke patients, but the technology to treat large vessel strokes is only available at select hospitals. Proper stroke-type identification and transport can shorten time to treatment. With the breakthrough designation, the FDA is recognising the potential for improving outcomes with this patient group.”