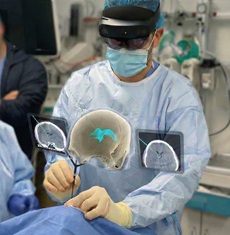
Medivis has announced that it has received US Food and Drug Administration (FDA) 510(k) clearance for its cranial navigation platform, making it the “world’s first” augmented reality (AR) system cleared for intraoperative guidance in cranial neurosurgery, according to the company. This marks Medivis’ second major US FDA clearance this year following the launch of its spine navigation platform in April.
By using AR to spatially map patient imaging within the operative field, the Medivis platform gives surgeons a clear, real-time view of critical anatomy and planned trajectories—an approach that can support faster, more confident decision-making during cranial procedures while minimising workflow disruption and reducing dependence on external monitors. The platform’s portable design enables reliable image guidance in settings where conventional systems fail—especially the intensive care unit (ICU)—extending image-guided precision to a wider range of clinical environments, Medivis claims in a press release.
The company also notes that, today, external ventricular drains (EVDs) are misplaced at rates reported to be as high as 30%, often leading to repeated passes, patient harm, and delayed critical care. By providing real-time, AR-guided visualisation at the bedside, early clinical experience suggests Medivis can significantly reduce these misplacements, directly improving patient safety, accelerating life-saving interventions and raising the standard of care across neurosurgery. Surgical intelligence, ergonomic freedom and seamless integration are listed by Medivis as key benefits of its newly cleared cranial navigation platform.
“For the first time, neurosurgeons can perform cranial procedures using AR—merging the digital and physical worlds with high-accuracy guidance,” said Osamah Choudhry, chief executive officer (CEO) and co-founder of Medivis. “This is a profound milestone not only for Medivis but for the entire field of neurosurgery. With this clearance, we’re bringing image-guided navigation to the ICU, where it hasn’t been possible before, giving clinicians greater precision at the bedside and helping support safer care for patients while paving the way for full integration into operating rooms.”
“This achievement reflects an extraordinary collaboration between our team and the US FDA, whose leadership and shared commitment to elevating patient care made this innovation possible,” said Christopher Morley, president and co-founder of Medivis. “This milestone not only attests to our technology’s capabilities but also lays the foundation for broad deployment of AR guidance across ICUs, operating rooms and surgical centres worldwide—advancing a future where surgical intelligence improves outcomes in every clinical setting.”








