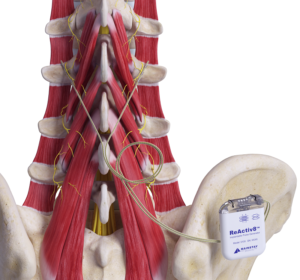
 Mainstay Medical has announced that the US Food and Drug Administration (FDA) has approved the company’s premarket approval (PMA) application for ReActiv8, its implantable neurostimulation system to treat intractable chronic low back pain.
Mainstay Medical has announced that the US Food and Drug Administration (FDA) has approved the company’s premarket approval (PMA) application for ReActiv8, its implantable neurostimulation system to treat intractable chronic low back pain.
“ReActiv8 is designed to be a restorative treatment and represents a new option for patients suffering with chronic low back pain. This disease affects millions of people around the world, and our clinical data demonstrates that ReActiv8 therapy provides progressive improvements in pain and disability over time, both in magnitude of effect and the proportion of patients who benefit from the treatment. This therapy has the potential to improve quality of life for the most severely-affected patients, and we look forward to making it available to US patients and physicians beginning in the first half of 2021,” commented Jason Hannon, CEO of Mainstay, in a company press release.
Chris Gilligan, principal investigator of the ReActiv8-B study, added: “ReActiv8 fills an unmet clinical need of patients suffering from chronic low back pain. Patients indicated for ReActiv8 therapy have generally tried numerous other treatments, including physical therapy and pain medications, and many are on long-term opioids to manage their pain.
“I have seen ReActiv8 provide durable improvements in back pain, the disabling effects of back pain, and quality of life. I am proud to have served as Principal Investigator of this landmark trial, and I look forward to sharing this experience with my physician colleagues who want to start using ReActiv8 in their patients.”
Robert Levy, a prominent neurosurgeon and president of the International Neuromodulation Society (INS), commented: “The use of neuromodulation to target underlying functional and motor-control issues in patients with musculoskeletal back pain can address a large unmet clinical need. ReActiv8 is designed as a restorative therapy for those suffering from musculoskeletal pain and does not compete with other forms of neuromodulation such as spinal cord stimulation. These patients are difficult for clinicians to treat with current therapy options, which is why so many of them take opioids to manage their pain.”
The FDA approval of ReActiv8 is primarily based on results from the ReActiv8-B clinical study, a pivotal 204-patient, international, multicentre, prospective, randomised, active sham-controlled, blinded trial with one-way cross-over, conducted under an investigational device exemption (IDE) from FDA.










