
Jason Hannon has been announced as successor to Peter Crosby as chief executive officer of Mainstay Medical, with effect from October 9, 2017. According t oa company release, this move was planned in association with Crosby’s upcoming retirement, due to take place at the end of October.
Hannon most recently served as president and chief operating officer of NuVasive. Prior to this, he served in roles at the company including executive vice president of International, executive vice president of Business Development and Strategy, and general counsel.
Hannon says: “Mainstay has developed a strong foundation in its scientific, clinical and regulatory accomplishments to date. The dedicated team has done the pioneering work to establish a new market—ReActiv8 seeks to help the body repair itself rather than merely masking pain. This has created the potential of bringing an entirely new option to people suffering from chronic back pain. I am impressed by the work done to get to this point, and I look forward to working with the entire team to advance the mission.”
In the period up to his retirement date, Crosby will act as special adviser to the new chief executive officer, and will continue to work with the company as a consultant up to the end of 2020. He will also continue to act as a director until the conclusion of the company’s Annual General Meeting to be held on Friday, 22 September 2017, when he will retire as a director.
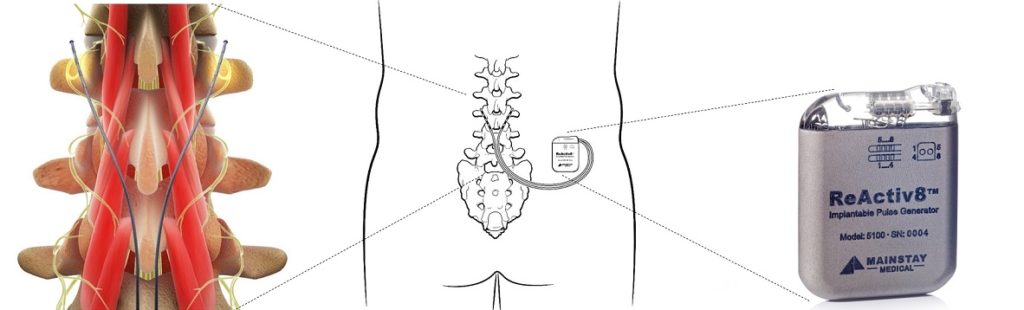
Crosby led Mainstay in its development of ReActiv8 from concept to commercialisation. He was recruited as the company’s first chief executive officer in 2009 to build the company and its team and to develop ReActiv8. Crosby led Mainstay through its Series A and Series B fundraisings to its IPO on Euronext Paris and the ESM of the Irish Stock Exchange in 2014, and its subsequent debt and equity fundraisings.
In addition to fundraising, Crosby aided in the development of ReActiv8 from concept stage through multiple clinical trials to CE mark approval in 2016, start of the ReActiv8-B clinical trial to gather data for US approval, and first commercialisation in Germany and Ireland in 2017.













