 Infinity Neuro announced today that its Inspira aspiration catheters have received CE-mark approval and are now commercially available in Europe.
Infinity Neuro announced today that its Inspira aspiration catheters have received CE-mark approval and are now commercially available in Europe.
This is the first offering from Infinity—a company that plans to launch a full range of products for the treatment of ischaemic and haemorrhagic stroke throughout 2023 and beyond, according to a press release.
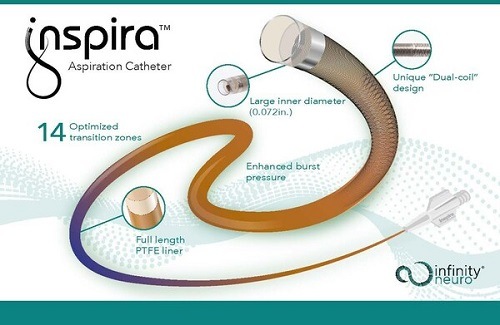
The Inspira aspiration catheters are “physician-inspired” to deliver next-level navigation and aspiration performance; designed to access targeted vessels easily, restore cerebral blood flow quickly, and retrieve clots in patients experiencing acute ischaemic stroke due to a large vessel occlusion (LVO).
As per the release, Inspira is the first phase of a complementary integrated system that will offer greater ease-of-use and performance with first-to-market product innovations. Additionally, this milestone represents the company’s commitment and ability to attain Class III medical device approvals through the new, rigorous European Medical Device Regulation (MDR)-based CE-certification process.
Infinity claims that this marks the first neurovascular device approved under the new European MDR CE-certification process as an original submission.
The Inspira aspiration catheter is indicated for injection of intravascular fluids; the introduction of interventional devices into the peripheral and neuro vasculature; and removal/aspiration of soft emboli and thrombi from the arterial system, including the peripheral and neuro vasculature.
The European commercial launch of these devices is now underway through a network of EU distributors.













