Route 92 Medical has announced the results of a “first-of-its-kind” multicentre study—published recently in Stroke: Vascular and Interventional Neurology—demonstrating the utility of the company’s Monopoint system in procedures to treat idiopathic intracranial hypertension (IIH). Physicians participating in the study noted that the Monopoint system successfully navigated tortuous patient anatomy and crossed the stenosis, offering improved trackability and sufficient catheter length to enable successful stent delivery.
Route 92 Medical has announced the results of a “first-of-its-kind” multicentre study—published recently in Stroke: Vascular and Interventional Neurology—demonstrating the utility of the company’s Monopoint system in procedures to treat idiopathic intracranial hypertension (IIH). Physicians participating in the study noted that the Monopoint system successfully navigated tortuous patient anatomy and crossed the stenosis, offering improved trackability and sufficient catheter length to enable successful stent delivery.
“We demonstrated that the flexibility of the Monopoint system, with a simple setup and a variable length, is extremely helpful in venous sinus stenting procedures where crossing stenosis to place venous stents can be challenging with less adaptable solutions,” said study author Oded Goren (Geisinger Medical Center, Danville, USA). “As a result of their venous sinus stenting procedures, study participants experienced a range of clinical improvements including a reduction in headaches, pulsatile tinnitus, and papilloedema.”
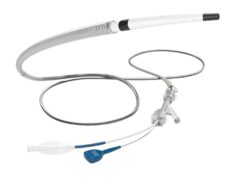
A Route 92 press release notes that this retrospective, multicentre, observational study aimed to understand the effectiveness of using the Monopoint system to access the venous sinus, cross through stenosis and allow delivery of a stent. The study included 71 patient procedures, which took place between January 2022 and December 2023 at 13 high-volume neurovascular centres in the USA. This is the first such study using the Monopoint system to facilitate venous sinus stenting, the company’s release also states.
“Physicians participating in the study rated the Monopoint system as better than conventional approaches 93% of the time,” noted Tony Chou, founder and chief executive officer (CEO) at Route 92. “The Monopoint system’s single axial setup and large bore catheter design makes it an ideal choice for complex neurovascular procedures, such as venous sinus stenting where physicians must navigate through tortuous anatomy and a stenosis to reach the target location.”
The Monopoint system is comprised of the Base Camp 8Fr sheath, HiPoint 88 catheter, and Tenzing 8 delivery catheter, which solved length compatibility problems that occur with conventional systems, Route 92 claims. The study showed that the Monopoint system could support the deployment of ‘relatively stiff’ stent systems—systems that benefit from a large bore catheter, which can reduce the need for complex multiaxial catheter access setups as well as over-the-wire exchange manoeuvres. The release further states that the variable catheter length of the Monopoint system and improved trackability through tortuous anatomy also supported procedural successes.
Matthew Alexander (Sutter Medical Center, Sacramento, USA) recently presented this study at the Society of NeuroInterventional Surgery (SNIS) Cerebral Venous and Cerebrospinal Fluid (CSF) Disorders Summit (19–21 March 2025, Honolulu, USA).
In its release, Route 92 clarifies that the stents referenced in this publication are not approved for venous stenting.