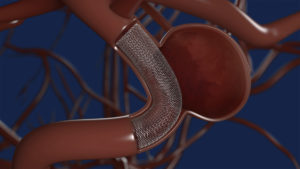
Medtronic has received US Food and Drug Administration (FDA) approval for the Pipeline Flex embolization device with Shield Technology for the treatment of aneurysms.
Medtronic’s Shield Technology was developed to advance flow diversion therapy by introducing a surface-modified implant device to reduce thrombogenicity of the device material.
The first US procedure with the new device was performed at NYU Langone Health in New York.
Peter Kim Nelson, chief of interventional neuroradiology and professor of radiology and neurosurgery at NYU Langone Health, said: “The Pipeline Flex-Shield that we used today at NYU Langone to treat a giant left internal carotid aneurysm represents a pivotal milestone in the evolution of flow diversion therapy—establishing a new vanguard for safe and effective management of complex cerebral aneurysms.
“Our team has long anticipated the availability of this device for patients in the United States. The surface modification of the implant has demonstrated reduced material thrombogenicity, discernibly aiding delivery through tortuous vascular anatomy with improved delivery and resheathing forces compared to earlier generation flow diverters.”
The Pipeline embolization device was first approved by the FDA in 2011. Pipeline was developed by Covidien, which merged with Medtronic in January 2011.
In June 2020, Hal Rice et al published results from the SHIELD study in the Journal of NeuroInterventional Surgery. The findings demonstrated safety and efficacy from periprocedural to one-year outcomes with the Pipeline embolization device with Shield Technology for intracranial aneurysms. The investigators reported 77.2% complete aneurysm occlusion at 12 months, a 3.2% primary safety endpoint, and 93.1% complete wall apposition post-procedure.