Rapid Medical has announced that it has completed enrolment in the TIGER (Treatment with Intent to generate reperfusion) study ahead of the planned scheduled. This is a USA-based, multicentre study of the performance of TIGERTRIEVER, the company’s thrombectomy device for the acute treatment of ischaemic stroke.
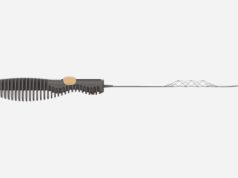
The TIGERTRIEVER is a fully-visible, controllable stentriever that is adjusted to fit the dimensions of a blocked blood vessel causing acute ischaemic stroke. Jeffrey Saver, stroke neurology director at Ronald-Reagan-UCLA Medical Center in Los Angeles, USA, and neurointerventionalist Rishi Gupta director, Neurocritical Care at WellStar Health System, Marietta, USA, are the principal investigators of the study.
TIGER is an investigational device exemption study evaluating the safety and effectiveness of Rapid Medical’s TIGERTRIEVER for treatment of ischaemic brain stroke. The study results will be used as part of the 510K submission of the device for US Food and Drug Administration (FDA) clearance.
The study was completed ahead of schedule and took place at 16 of the leading stroke centres throughout the US and one centre in Israel. The TIGERTRIEVER device has CE mark clearance and is commercially available in Europe; thousands of ischaemic stroke thrombectomy procedures are performed with the device every year.
“The TIGERTRIEVER is a new generation stentriever which provides the physician with enhanced user control. Its unique design will hopefully show that it addresses the limitations of current devices to provide optimal patient outcomes. We are looking forward to publishing the data,” stated Gupta.
“It was our honour to co-lead this trial and we would like to thank the patients, their families, and the clinical sites who participated in the study. Their dedication enabled rapid patient recruitment and trial completion ahead of schedule, despite the extra challenges of the current medical moment,” said Saver.