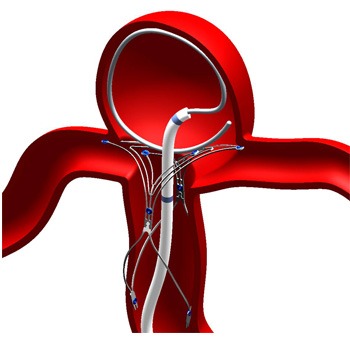
Pulsar Vascular has announced that they have reached the target enrolment in their clinical trial for PulseRider device for the treatment of intracranial aneurysms. This multicentre study was conducted under an Investigational Device Exemption (IDE) granted by the US Food and Drug Administration (FDA) at 10 centres of excellence nationwide.
The principal investigator for the study, is Alejandro Spiotta, assistant professor of Neurosurgery and Interventional Neuroradiology, at the Medical University of South Carolina.
PulseRider is a medical device designed to address an unmet need in treating complex aneurysms of the brain. Rob Abrams, the president and chief executive officer of Pulsar Vascular said, “Completing enrolment of the clinical trial highlights an important milestone for the company. We now look forward to the next steps, which include patient follow-up and the analyses of data in support of PulseRider, which we anticipate will be reported to FDA early in 2016.” Abrams also reported that, “We are delighted with our progress in Europe and Japan, treating these complex lesion.”
PulseRider is not available for sale in the United States. This device is limited by United States law to investigational use only.












