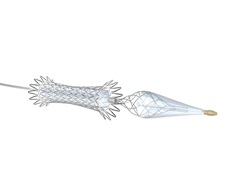
Contego Medical today announced the US Food and Drug Administration’s (FDA) premarket approval (PMA) of the Neuroguard IEP system for carotid revascularisation.
The company shares in a press release that clinical studies of the Neuroguard IEP system, including the PERFORMANCE I trial and PERFORMANCE II investigational device exemption (IDE) trial, have consistently recorded “unprecedented” low event rates—zero major strokes, zero neurologic deaths, and zero stent thrombosis at 30 days and one year.
William Gray, system chief of the Division of Cardiovascular Diseases at Main Line Health (Wynnewood, USA) and co-national principal investigator of the PERFORMANCE II trial, said in the press release: “FDA approval confirms the results of the clinical studies. The innovative Neuroguard IEP system performs exceptionally well with the lowest one-year stroke rates ever shown for any type of carotid revascularisation, thereby establishing a new standard of care for meaningfully reducing the risk of procedural and long-term stroke among patients with carotid artery disease.”
The Neuroguard IEP system utilises Contego Medical’s Integrated Embolic Protection (IEP) technology, featuring a 40-micron filter integrated on a 6Fr delivery catheter. The size of the integrated filter can be adjusted by the operator to custom fit each patient’s unique anatomy, and the pores on the filter are three to four times smaller than those on traditional filters, the company claims, improving protection against stroke and cognitive impairment. According to Contego Medical, the newest generation closed cell stent is highly conformable and shows remarkable short- and long-term durability, with no thromboses, no clinically driven target lesion revascularisation, and very low restenosis rates at one year.
“This FDA approval is a huge step forward for Contego and for patient care. The Neuroguard IEP system transforms how we approach patients with carotid artery disease by addressing the specific threats of microembolisation while simultaneously reducing procedural steps, ensuring patients receive the highest level of protection throughout the procedure,” said Ravish Sachar, Contego Medical’s chief executive officer and founder. “The ongoing PERFORMANCE III trial is evaluating the Neuroguard IEP system with a next-generation direct transcarotid access and protection system, TCAR-IEP, to demonstrate advanced stroke protection regardless of vascular approach.”
In 2023, the Centers for Medicare and Medicaid Services (CMS) issued a national coverage decision significantly expanding coverage for percutaneous transluminal angioplasty (PTA) of the carotid artery concurrent with stenting performed with FDA-approved carotid stents and embolic protection devices. Contego Medical states that this decision and FDA approval of the Neuroguard IEP system positions the company for ongoing growth.










