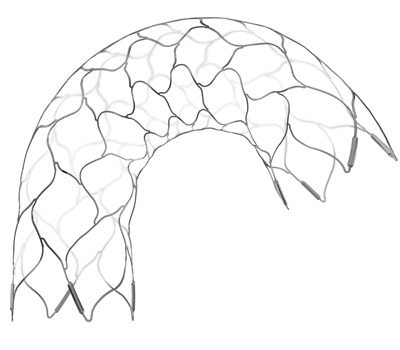
Codman Neuro has launched the Enterprise 2 Vascular Reconstruction Device, the latest generation of the company’s self-expanding stent and delivery system used to treat wide-necked intracranial aneurysms and to help maintain the position of endovascular coils during and after the procedure.
The new device was featured at AANS/CNS Joint Cerebrovascular Annual Meeting in Los Angeles, USA, held in conjunction with the Society for Neurointerventional Surgeons (SNIS).
The new Enterprise 2 System is designed to improve vessel wall conformability, while maintaining a stable structure at the neck of an aneurysm. The device helps secure the placement of endovascular coils and maintains blood flow through the artery. In addition, the stent is more visible under fluoroscopy than the previous device and has a self-flushing introducer to facilitate ease of use.
“The precision, conformability and occlusion that can be achieved when treating wide-necked aneurysms with the Enterprise 2 System are excellent. It was easy to use and deploy, and met all expectations for treatment. This is truly a next generation stent that helps overcome the clinical challenges of treating wide-necked aneurysms,” said Donald Frei, a neurointerventional radiologist at Radiology Imaging Associates in Denver, USA, and one of the first physicians to use the technology.
According to the American Stroke Association, about three to five million people in the United States have some form of brain aneurysm, though most do not produce any symptoms. However, between 0.5 and 3% of people with a brain aneurysm may suffer from bleeding and rupture and require treatment. If an aneurysm has not ruptured, the treatment decision depends on its size, location and shape, and the patient’s symptoms.
“We have made important design improvements to the Enterprise 2 System so that it better fits vascular anatomy, is more visible on X-ray, and is more easily deployed,” said P Laxmin Laxminarain, worldwide president of Codman Neuro. “This technology is specifically designed to enhance the way physicians repair wide-necked aneurysms, which can be life-threatening and very difficult to treat.”
The Enterprise Vascular Reconstruction Device and the Enterprise 2 Vascular Reconstruction Device are Humanitarian Use Devices approved by the FDA under a Humanitarian Device Exemption (HDE) in the United States Only, where it is authorised by Federal Law for use with embolic coils for the treatment of wide-neck, intracranial, saccular or fusiform aneurysms arising from a parent vessel with a diameter of ≥2.5mm and ≤4mm. Wide-neck is defined as having a neck width ≥4mm or a dome-to-neck ration <2.









