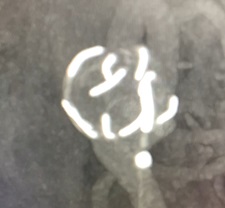
Arissa Medical has announced six-month digital subtraction angiography (DSA) follow-up of the first cohort of patients in its Syntra first-in-human (FIH) study—an early feasibility study evaluating the safety and efficacy of the company’s Syntra device for the treatment of wide-necked sidewall and wide-necked bifurcation cerebral aneurysms.
The six-month radiographic follow-up in the study was performed by independent medical monitor Naoki Kaneko (University of California Los Angeles [UCLA], Los Angeles, USA) and—according to a press release from Arissa—this clinical report summary from Syntra FIH provides evidence of a favourable safety and efficacy profile.
The Syntra FIH study’s six-month follow-up results initially indicate that the company’s proprietary intrasaccular scaffold device is a safe and effective treatment for wide-necked intracranial aneurysms. In addition, the absence of serious adverse events coupled with observations of successful occlusion rates align with the study’s safety and efficacy goals. However, the company’s recent release also notes that results from the final 12-month DSA follow-up are still needed in order to assess more long-term outcomes.
The site for the Syntra FIH study is Instituto de Neurocirugia Alfonso Asenjo in Santiago, Chile, and the study’s interventional neuroradiology (INR) principal investigator is Juan Gabriel Sordo.









