Acandis has announced that it has received approval from the French national competent authority for medicines and health products, Agence Nationale de Sécurité du Médicament et des Produits de Santé (ANSM), and the responsible ethics committee to initiate the RESOLVE clinical study.
This pivotal, multicentre, international clinical investigation will evaluate the company’s Sirex stent for the treatment of pulsatile tinnitus caused by symptomatic lateral venous sinus stenosis, with the first study initiation scheduled to take place in early August.
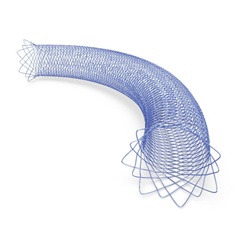
RESOLVE has been designed to assess the safety, efficacy and clinical benefit of the Sirex stent—a novel, braided and fully radiopaque implant engineered specifically for the unique anatomical and clinical challenges of venous sinus stenting (VSS).
A total of 78 patients will be enrolled across five European centres, with the study coordinated by Emmanuel Houdart (Lariboisière Hospital, Paris, France), a leading expert in the VSS field.
Pulsatile tinnitus—an often-debilitating condition characterised by a sound synchronised with the heartbeat—is frequently caused by stenosis of the lateral venous sinus. A press release from Acandis notes that, while clinicians like Houdart have successfully treated this condition for years using available stents originally developed for arterial use, Sirex represents an “important next step”, offering a solution specifically tailored for venous applications. Its advanced features aim to make treatment more accessible, safe and reliable for a broader patient population.
“The possibility to treat pulsatile tinnitus via stenting has already changed lives,” Houdart commented. “What sets the Sirex stent apart is that it is purpose-built for this indication. It combines technical specifications, such as flexibility, full radiopacity and a dedicated, small delivery system, to make the procedure safer and more consistent.”
The RESOLVE study’s main objective is to demonstrate both performance and safety of the device. Its primary clinical endpoints include the disappearance of pulsatile tinnitus at 90 days and confirmation of stent patency via venous cerebral computed tomography (CT) angiography. Safety will be measured by the rates of device- and procedure-related complications, as well as device-related serious adverse events at 90 days, 12 months, and 24 months.
“Launching the RESOLVE study marks a major milestone for Acandis in addressing unmet clinical needs,” said Andreas Schüßler, founder and chief executive officer (CEO) of Acandis. “The Sirex stent reflects our commitment to innovation in neurovascular care—designed from the ground up to treat patients with lateral sinus stenosis more effectively and safely.”
Enrolment in RESOLVE is expected to conclude within 24 months, with follow-up continuing for an additional two years. Results from the RESOLVE study are intended to support CE certification of the Sirex stent, and expand its clinical use across Europe and beyond, Acandis also states.









