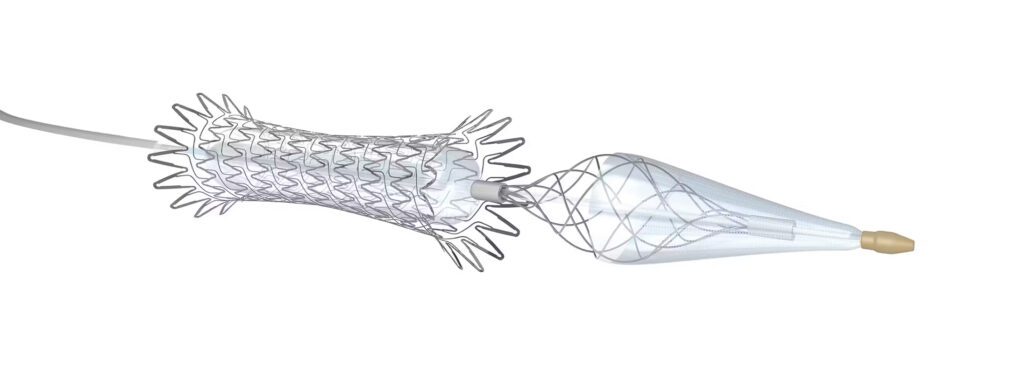
Medtronic has announced the full distribution of the Neuroguard integrated embolic protection (IEP) system for carotid artery disease following its limited market distribution. This follows the announcement earlier this year of an exclusive US distribution agreement between Medtronic and Contego Medical, the developer of the device.
Neuroguard is marketed as the first carotid stent to offer a three-in-one technology, combining a stent, post-dilation balloon and filter.
Data from the PERFORMANCE II study of the device demonstrated no major strokes, neurological deaths, stent thromboses or clinically driven target lesion revascularisation—despite a 34.5% rate of severely calcified lesions—at 30 days and one year, the company stated in a press release.
Contego Medical is currently evaluating the Neuroguard IEP system with a next-generation direct transcarotid access and protection system, TCAR-IEP, to demonstrate safety and effectiveness in the PERFORMANCE III trial.