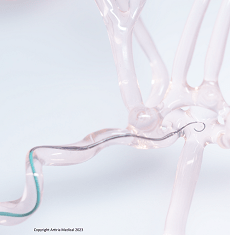
Artiria Medical, a Swiss neurovascular company, has announced that its real-time, deflectable guidewire has been granted 510(k) clearance by the US Food and Drug Administration (FDA).
This milestone opens a “new pathway” to a “more advanced and effective approach” to stroke treatment, and related neurovascular and peripheral conditions, as per a company press release.
Artiria’s guidewire offers “the next level of control” for physicians as well as an improved way to navigate the complex network of the cerebral arteries, the release adds. The distal tip of the device can be shaped, in real time, without it being removed from the patient, while the support profile of the wire can be adjusted over the duration of a procedure, as needed.
“We are excited about receiving the FDA clearance on a cutting-edge, Swiss-made technology,” said Guillaume Petit-Pierre, co-founder and CEO of Artiria. “We are looking forward to starting our clinical activities in the USA very soon.”
“Our sincere thanks go out to our dedicated team who has helped us to reach this significant milestone,” added Marc Boers, co-founder and COO of Artiria.
Neurovascular interventions are often hindered by the time-consuming task of navigating through the dense maze of the brain’s arteries, Artiria states in the release—adding that its latest innovation provides a novel solution to this that can be seamlessly integrated into existing clinical practices.
As per the release, next steps for the company involve gaining clinical experience through collaboration with USA-based centres in order to demonstrate the clinical effectiveness of its newly approved product.









