By Julie Staals
Cerebral small-vessel disease (SVD) is a prevalent age-related disease of the brain. It is a common cause of ischaemic (lacunar) stroke and deep haemorrhagic stroke. It may also lead to gait disturbances, cognitive impairment and even dementia. It has growing impact on the ageing society and health care system worldwide. Age and hypertension are considered the most important risk factors. There are multiple gaps in our knowledge about the pathogenesis and even the ‘standard’ vascular preventive treatments have no convincing proven benefit for cerebral small-vessel disease.
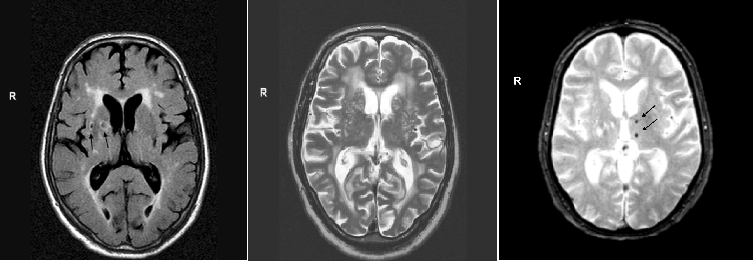
Radiologically, the cerebral small vessels cannot be visualised. The structural consequences on the brain parenchym are therefore used to study the underlying small vessel disease. These structural features that can be seen on conventional MRI are: lacunes, white matter hyperintensities, perivascular spaces in the basal ganglia, and cerebral microbleeds. They are considered ‘MRI markers’ for cerebral small vessel disease (Figure).
One of the reasons for the insufficient knowledge about cerebral small-vessel disease is that research always focused on individual radiological features, ignoring the co-occurrence of these features. We think that an imaging scale that integrates all four MRI features of cerebral small-vessel disease would better capture the overall burden and impact of the disease and would improve the assessment of disease severity.
Recently, the Maastricht neurovascular research group proposed a simple method to assess the overall burden of cerebral small-vessel disease: one point is assigned for the dichotomised presence of each of the four MRI markers, adding up to a 0 to 4 composite SVD score.
To further validate this total SVD score, the Edinburgh research group tested in a large ischaemic stroke sample (n=461 patients) whether the SVD score was associated with previously described vascular risk factors for individual SVD features. We also tested whether SVD score was higher in patients with lacunar (small vessel) stroke compared to cortical (large vessel) stroke. Our results were recently published in Neurology.
We showed that higher total SVD score was associated with age, male sex, hypertension, smoking and lacunar stroke subtype. Furthermore, lacunar stroke patients had higher ratings of SVD burden compared with cortical stroke patients.
The score provides a more complete overall view of the impact of small-vessel disease on the brain than do the individual MRI features separately. It is a simple and pragmatic visual quantification of cerebral small vessel disease that can easily be performed in clinical practice. For research purpose, the total SVD score could be helpful for baseline stratification or as a surrogate marker for small vessel disease in prevention and therapeutic trials. In clinical practice, it could be used to identify subjects at risk of stroke, cognitive decline and physical impairments, although further studies for refinement and validation of the score are needed. We hope that the implementation of the SVD score will contribute to a better understanding of cerebral small-vessel disease, in the end leading to better treatment strategies.
Julie Staals worked with the Brain Research Imaging Centre, Edinburgh, UK, and is currently at the Department of Neurology and Cardiovascular Research Institute Maastricht at Maastricht University Medical Center, the Netherlands













