The final results of the first thrombectomy trial in a developing country convey the “overwhelming efficacy of mechanical thrombectomy”. Given that 35% of the treatment arm achieved a modified Rankin Scale (mRS) score of 0–2 compared to 20% of controls, Raul G Nogueira (Grady Memorial Hospital, Atlanta, USA) put forward: “We have overcome financial, logistical and political barriers [and], by doing so, we have had the opportunity to impact and influence many other countries that share our same reality.” The data from the RESILIENT (Randomisation of endovascular treatment with stent-retriever and/or thromboaspiration versus best medical therapy with acute ischaemic stroke due to large vessel occlusion) trial were presented by Nogueira and Sheila Martins (Universidade Federal do Rio Grande do Sul, Porta Alegre, Brazil) at the European Stroke Organisation Conference (ESOC; 22–24 May, Milan, Italy).

Addressing the ESOC audience, Nogueira stated: “It took exactly 17 years for the government [of Brazil] to begin paying for tPA in the public heathcare system… this is the reality of healthcare outside of first-world countries. The issue is that although we are dealing with the second cause of death and the main cause of disability… the cost of thrombectomy was judged to be too expensive for the country”.
He further alluded to the array of randomised clinical trials that have consistently demonstrated the benefit of mechanical thrombectomy for the treatment of large vessel occlusion stroke. However, he highlighted the fact that these studies were performed in “high-income” countries, and said, “they have had minimal public healthcare impact on low- and mid-income countries”.
Providing an explanation as to why thrombectomy outcomes might differ in countries with lower resource, and specifically, why the current study was likely to elicit negative results, he said that in the prehospital setting, delays in diagnosis, transfer, and patient triage are common, while he added, “Our population is also a lot more vulnerable in terms of nutrition, socio-economical and educational status”. Regarding the hospital infrastructure itself, he speculated that “different training of neurointerventionalists, a difference in available angiosuite technology, post-procedural treatment, and a lack of access to rehabilitation” all further contribute to suboptimal thrombectomy outcomes.
Despite these limitations encountered in the public healthcare system of Brazil, the Ministry of Health agreed to sponsor a thrombectomy trial. Co-chaired by Nogueira and Matins, the study investigators carried out a multicentre, prospective, randomised, open, blinded endpoint, controlled trial in 20 stroke centres in Brazil.
The patient population (n=690) consisted of those with acute ischaemic stroke with large vessel occlusion (ICA and/or MCA-M1) no more than eight hours from onset. Specifically, patients had a disability of modified Rankin Scale ≤1, while further key inclusion criteria included NIHSS ≥8 and ASPECTS ≥6 (or ≥5 on diffusion-weighted imaging MRI [DWI MRI]) at baseline. Patients were randomised 1:1 to either Solitaire (Medtronic) and/or Penumbra plus medical management, or medical management alone. Follow-up took place after 24 hours, at discharge and on day 90.
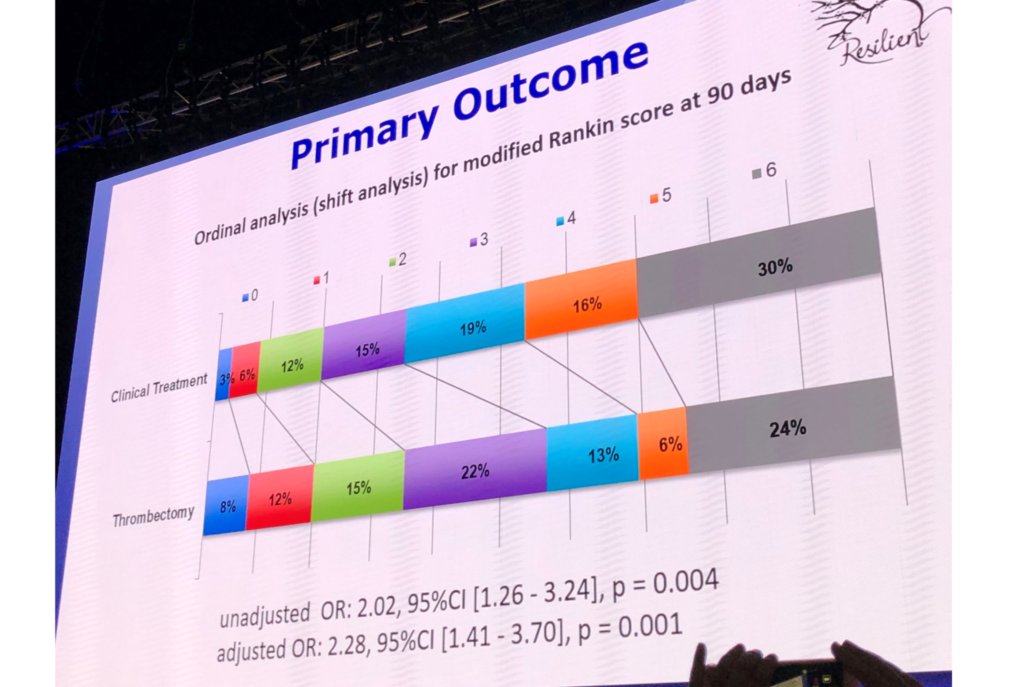
The study investigators set the primary endpoint as the distribution of the 90-day mRS scores (ordinal-shift analysis) as evaluated by a blinded core lab through video interviews.
Although the sample size was set to achieve 690 patients for a 10% treatment effect difference, on the 3rd of March earlier this year, the Data and Safety Monitoring Board (DSMB) recommended that the trial should be terminated due to a “clear crossing of the boundary for efficacy at the first interim analysis” after 174 patients had completed 90-day follow-up.
Thus, in total, 221 patients with “similar baseline characteristics” were randomised over the course of 25 months: 111 to mechanical thrombectomy and 110 controls (average age: 64, mean NIHSS: 18±5, 65% received tPA, 18% with ICA occlusions).
In terms of patient presentation and procedural duration, symptom onset-to-needle time was below three hours in both groups: 170 (range: 132–213) minutes for thrombectomy and 161 (115–219) minutes for controls. Door-to-needle times were 34 (25–53) and 33 (25–50) minutes for thrombectomy and controls, respectively, while door-to-puncture time was 116 (90–159) minutes. Finally, symptom-to-recanalisation times were around five hours, taking an average of 317 (273–390) minutes.
Turning to the ESOC audience, Martins said that the ordinal analysis for the modified mRS score at 90-days displayed superiority for thrombectomy (adjusted OR: 2.28; 95% CI: 1.41, 3.7; p=0.001). Moreover, the secondary outcomes examining functional independence (mRS ≤2) at 90-days showed favourable results, as 35% of the thrombectomy arm achieved mRS 0–2, with only 20% of controls achieving the same result. This meant that the number needed to treat for one additional 90-day independent outcome was 6.6.
In terms of safety outcomes, Martins reported the incidence of symptomatic intracranial haemorrhage as low, at around 6–7% for patients that received thrombectomy, and 5–6% for controls. “The mortality was similar in both groups, with severe dependency [mRS 5–6] at 90-days higher in the clinical treatment arm: 46% versus 31% for the treatment arm,” added Martins. Only 12 patients underwent procedural complications.
Concluding the study, Martins reiterated: “Disability was significantly decreased in patients treated with mechanical thrombectomy (adjusted OR: 2.3) with improved functional independence at 90-days post stroke when compared to medical management alone.” In light of these data, she said, “The overwhelming efficacy of mechanical thrombectomy persists despite the many limitations encountered in the public healthcare system of a developing country.”
Thus, the final results of the current study; the first thrombectomy trial in a developing country, led Martins to put forward: “Mechanical thrombectomy should be available to many more patients… globally.”