The volumetric impedance phase shift spectroscopy (VIPS) device is a portable, noninvasive way of detecting severe stroke, including emergent large vessel occlusion (ELVO), with a sensitivity of 93% and a specificity of 92%, making it a promising tool for the triage of severe stroke patients. In contrast, a standard physical examination achieves an accuracy of 40–89% in identifying patients with large vessel occlusion who could benefit from endovascular therapy. The results of the VITAL study were published in the Journal of NeuroInterventional Surgery.
Current triage efforts consist mainly of neurological assessment that use physical examinations but these are highly user dependent and can fall short of sufficient diagnostic accuracy. Commonly used tests are the three-item Stroke Scale (3ISS), Los Angeles Motor Score (LAMS), Rapid Arterial Occlusion Evaluation Scale (RACE), the Cincinnati Prehospital Stroke Severity Scale (CinPSS), Field Assessment Stroke Triage for Emergency Destination (FAST-ED), and PASS. These scales focus on different elements of the neurological examination. Some put accuracy first while others focus on reproducibility and ease of use. Their diagnostic accuracy remains low, with reported sensitivities ranging from 55% to 85%, specificity from 40% to 89%, and area under the curve from 0.73 to 0.78.
The authors write, “Given the complexity of presentations associated with neurologic disease and the high rate of severe stroke mimics, scales that rely entirely on the neurologic examination are inherently error prone and incapable of sufficiently diagnosing severe stroke patients.”
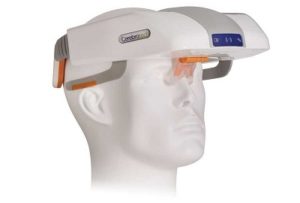
VIPS (Cerebrotech Medical Systems) is a non-invasive technology capable of detecting a bioimpedance signature across body tissue. The device functions by transmitting an array of varying frequencies of low energy radio waves from each side of the back of the head to a receiver in the forehead portion of the visor. Radio waves of different frequencies are modified differently as they pass through the tissue, depending on the type and fluid properties of the tissue, producing a unique signature for varied brain pathologies and the VIPS device exploits the alterations in tissue electrical properties that occur as a result of fluid and electrolyte changes. In a region of the brain that is ischaemic due to an ELVO, arterial blood content is diminished and the distribution of fluids and electrolytes between the intracellular, extracellular, and intravascular spaces may be substantially shifted. These alterations result in a change in the tissue bioimpedance. Because acute stroke typically affects the hemispheres unequally, ELVO induces an asymmetry in the bioimpedance of the brain. The larger the fluid and electrolyte changes in the affected cerebral region (i.e., the larger the stroke), the greater the bioimpedance asymmetry.
The study showed that a novel non-invasive device can rapidly acquire physiological data that may yield high sensitivity and specificity for detection of severe stroke.
Stroke treatment systems of care have been designed for the past 20 years around rapid administration of tissue plasminogen activator. Now with level 1a evidence in support of thrombectomy for ELVO, it is important to be able to triage ELVO patients to thrombectomy capable centres as accurately and quickly as possible. Data suggest that approximately 75% of ELVO patients are denied a chance at thrombectomy due to delays in triage. The VIPS device is quick and easy to use, meaning it could significantly reduce triage times. Patients who are transferred from one hospital to another have longer revascularisation times, worse Alberta Stroke Program Early CT (ASPECT) scores at the time of treatment, and suffer worse outcomes.
The ideal diagnostic test to improve patient triage would be portable, fast, require minimal specialised training, pose no additional risks to the patient, perform in a reproducible and accurate manner, and be low cost such that it could be widely adopted in all ambulances to integrate into our current triage system. This is the first formal study evaluating a device with the potential to satisfy all of these characteristics.