

The in silico trials for treatment of acute ischaemic stroke (INSIST) consortium began in 2017 and presented its concepts and first results at the joint European Stoke Organisation and World Stroke Organization (ESO-WSO) virtual conference (7–9 November). NeuroNews had an exclusive interview with two of the project’s leaders, Charles Majoie (professor of neuroradiology at the Amsterdam University Medical Center Amsterdam, The Netherlands), co-principal investigator (PI) of the MR CLEAN trial, the MR CLEAN registry, and now co-PI of the MR CLEAN NO IV trial and INSIST, and Alfons Hoekstra, professor of computational science and engineering, director of the Informatics Institute at Amsterdam University (Amsterdam, The Netherlands) and co-PI of INSIST.
Majoie explained the aim of their project, “Performing a randomised control trial (RCT) is a huge burden and also very expensive, for researchers as well as patients. Despite good designs, they often fail. So, with INSIST, we aim to generate a platform to perform the trials in silico through computational modelling and simulation technologies. We now have computer simulations, which are standard technology in many industries like automotive, aeronautics or engineering, but they are really underestimated for the health care setting. INSIST is based largely on existing databases like the MR CLEAN trial and MR CLEAN registry. We have generated a virtual population model, which is an effective covariant distribution model, incorporating all relevant patient parameters that may account for the differences between those individuals. We also generated models to simulate thrombosis and thrombolysis, thrombectomy, and models simulating tissue fate during and following ischaemic stroke. We have also generated a platform for in silico trials.”
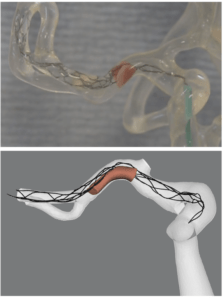
Hoekstra added, “We are talking about mathematical models. What we do is try to mimic parts of physiology in the computer and then we are able to do that. We can also model the path of physiology in a computer, in this case a stroke, and we can also model treatment. We have a movie showing a thrombectomy treatment, but this is not an animation. This is completely computed from the basic laws of physics and from the basic laws of physiology and biology. And that is one big thing to realise. And so when we speak about models, this is what we mean.”
 Hoekstra continued with some ideas of where the project is now, “a big part of our work is to turn trial data, into real information for the INSIST project. We are getting close to being able to claim that our models are valid, meaning that indeed they represent reality and in this case, the stroke effect and the treatment effect. We are now getting ready to put all our models together to mimic the MR CLEAN trial. So we are basically trying to, in a retrospective sense, recreate the MR CLEAN trial in the computer.
Hoekstra continued with some ideas of where the project is now, “a big part of our work is to turn trial data, into real information for the INSIST project. We are getting close to being able to claim that our models are valid, meaning that indeed they represent reality and in this case, the stroke effect and the treatment effect. We are now getting ready to put all our models together to mimic the MR CLEAN trial. So we are basically trying to, in a retrospective sense, recreate the MR CLEAN trial in the computer.
If our in silico trial produces the same output as the original MR CLEAN trial, we have made a big step and we are quite optimistic that we are going to be able to do that. If that is the case, then we can open up a complete new territory. We can start to ask ‘what if’ questions to the computer. So, what if the MR CLEAN trial was a little bit different? What would be the outcome? If we repeat the MR CLEAN trial, but now with a different device, what would that look like? We need to try a few ideas that came up in the clinical context before putting that into a real randomised trial. That’s where we are going with this technology.
It will not tell you anything yet about the fate of an individual patient. We are not claiming we can predict that. This may happen in the future, but that is not the goal of this project. This technology will never fully replace trials. That is impossible. It will help to improve trials. It will help speed up trials.”
Majoie added what some of the benefits of this can be for clinicians, “I think that by trying to simulate thrombectomy, you also get more insight on what is really happening. Every detail is then incorporated in the model, the mechanical properties of the clot, and of the stent itself. So, you can see very good interaction between the stent and the clot. You have got more insight on what is really happening and then you get an idea how to optimise the device to improve the treatment. You can also adapt the design of the trial that you are planning to execute, when you first run it in silico, you could see the problems and the possible failures.”
Hoekstra commented on some obstacles they face, “One big barrier is the certification and accreditation of all this technology. From our very first moment, we interacted with the US Food and Drug Administration (FDA), who are very open to these ideas, which was a big support for this research. This resulted in a lobby towards the European Commission that understood the importance of this technology and decided to start to fund these ideas. So, we got a first wave of money to support the idea of in silico trials.”
Hoekstra continued, “the FDA and the European Medicines Agency (EMA), have both declared that in silico techniques are feasible and should be able to perform primary evidence in trials or replace primary evidence for trials, or other experiments. That means that you need to set up a whole chain of protocols for in silico technology to be accredited by FDA or the EMA to allow a piece of software to generate this data.”
The INSIST project is supported by the European Union’s Horizon 2020 research and innovation programme (Grant No. 777072; www.insist-h2020.eu).













