 CereVasc has announced the treatment of the first patient in a study of its eShunt System, an investigational device intended to treat communicating hydrocephalus (CH).
CereVasc has announced the treatment of the first patient in a study of its eShunt System, an investigational device intended to treat communicating hydrocephalus (CH).
According to a company press release, this is the first minimally invasive treatment for CH. CereVasc states that the eShunt device offers the potential to result in significant benefits over current treatment which they claim is a half-century-old neurosurgical procedure associated with frequent failure, infection risk and high costs. CereVasc report study data will be available in 2021.
“For nearly 30 years, a groundswell of clinicians, patients, professional societies and government agencies have advocated aggressively for improvements to cerebral spinal fluid (CSF) shunts,” said Carl Heilman, co-inventor of the eShunt system, neurosurgeon-in-chief at Tufts Medical Center, chairman of its department of neurosurgery, Boston, USA.
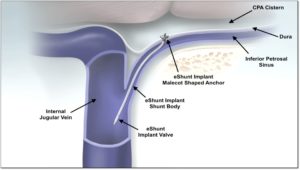
According to CereVasc, the eShunt System offers minimally invasive treatment for CH. The company states the eShunt is designed to eliminate the need for a craniotomy or surgical opening into the skull, passage of a catheter through white matter of the brain, subcutaneous tunnelling across the chest and abdomen for placement of a long outflow catheter into a second surgical incision in the abdomen.
The company further reports that in contrast, a clinician places the eShunt implant through a leg vein using X-ray guidance. CereVasc states that the minimally invasive approach brings the potential to dramatically reduce infection risk and several other common causes of ventriculoperitoneal shunt failure.
CereVasc claims that once in general use, clinicians will be able to deploy the eShunt implant in approximately one hour, potentially supporting an outpatient, day-surgery alternative to traditional neurosurgery.
“While attempts to address this call to action followed, CSF shunts continue to fail frequently, leading to unnecessary patient burdens and high costs.” Adel Malek, co-inventor of the eShunt system, director, cerebrovascular and endovascular surgery division at Tufts Medical Center, Boston, USA, continued, “We believe the eShunt System represents an opportunity to transform the quality and effectiveness of treatment for communicating hydrocephalus through a minimally invasive endovascular approach. The eShunt could improve patient outcomes and quality of life while potentially decreasing the high costs and risks associated with existing treatment.”
The ETCHES I (Endovascular treatment of communicating hydrocephalus with the eShunt system) trial is taking place at Clinica Sagrada Familia (Buenos Aires, Argentina) under the direction of principal investigator Pedro Lylyk (chair of neurosurgery and haemodynamics at the University of Buenos Aires, professor of the Department of Vascular Medicine at the University of El Salvador, and professor of Endovascular Surgery at the University of Ciencias Empresariales, Buenos Aires, Argentina). Lylyk commented: “Taking part in such an important trial with a device that has the potential to bring meaningful innovation to a half-century-old standard of care is thrilling for the entire team at Clinica Sagrada Familia here in Buenos Aires. We believe patients will experience significant benefits with the eShunt System. We are pleased to have our first patient treated and look forward to continuing the trial and generating data that will support the ongoing clinical evaluation of the System.”
“The failure rates for invasive brain surgeries to treat hydrocephalus patients are unacceptably high,” said Dan Levangie, chairman and CEO of CereVasc, Boston, USA. “The eShunt System has been designed to transform a half-century-old standard of care that we believe is in dire need of an overhaul. The ETCHES I study is a welcome development for clinicians, who have long sought a better way to treat CH. It is also a significant milestone for CereVasc, which supports our regulatory pathway and brings us closer to potentially delivering our novel device to a very deserving patient and clinician community.”
Diana Gray, president and CEO of the Hydrocephalus Association, Bethesda, USA, commented, “Hydrocephalus affects over one million people in the United States alone yet the primary treatment has remained the same for over 50 years. That is why we support the development of new innovations that could improve the lives of people living with this condition.”












Outstanding innovation