There is no doubt now that mechanical thrombectomy has been established as the new standard of care for the treatment of emergent large vessel occlusion stroke. But moving forward, stroke teams around the world have begun to look for ways in which the process can be improved so that the treatment can reach as many patients as possible, as quickly as possible. To do this, increasing focus is being placed on improving the pathways of care.
Mayank Goyal (University of Calgary, Canada), who has been at the forefront of pushing for speed and efficiency in stroke care, told the Society of NeuroInterventional Surgery annual meeting (SNIS; 25–28 July, Boston, USA) audience that in order to improve stroke workflow at any institution, the bottlenecks must be identified and eliminated. At his own institution, he and his team have gone through the process of expelling bottleneck after bottleneck on the journey to perfecting their own workflow.
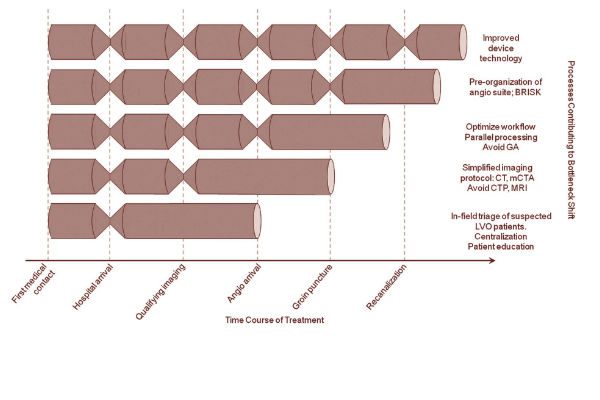
“Seven or eight years ago, we had all these bottlenecks in stroke care. Around 2009 was the first case I did with a stent retriever. Since then, device technology has improved and we got rid of that bottleneck; after that we found a bottleneck that it took a long time to get the angio staff going and organised, so I started working on that, came up with the idea of BRISK (Brisk Recanalisation Ischaemic Stroke Kit—a stroke tray ready to go with everything available) and we got rid of that bottleneck. We then decided that we needed to divide up the team and do parallel processing and avoid general anaesthesia and got rid of that bottleneck. After this, we decided that we needed to fix the imaging and we stopped doing CT (computed tomography) perfusion and MR (magnetic resonance) imaging and came up with the idea of doing multi-phase CT angiography and we got rid of that bottleneck,” Goyal revealed.
Justifying his chosen pathway of care, Goyal maintains that the only thing that is currently proven to work in the treatment of acute ischaemic stroke is the opening of the occluded vessel. The rest, he said, are just steps along the way. For example, Goyal explained, determining the size of the clot does not influence patient outcome; knowing that the NIHSS (National Institute of Health Stroke Scale) is 17 or 19 does not improve patient outcome; it is only opening the vessel that influences patient outcome.
“Here is a way to think about it—the onset to imaging decides the likelihood of favourable imaging, and the imaging to reperfusion decides the likelihood of favourable outcome. What does that mean in real terms? It means, if you imagine 100 patients 30 minutes from onset, nearly everyone would be eligible, if you get 60% good outcome, 60 out of 100 have a good outcome. If you take the patients at seven hours, maybe 10 of them have good imaging, and if you have 60% good outcome, six out of 100 have a good outcome. It is extremely important to remember that onset to imaging decides the likelihood of good imaging—that is sometimes forgotten and we may continually keep making this mistake of reporting by using the denominator of the number of patients we took to the cath lab,” he said.
Goyal advised other stroke teams to go through their own practice and figure out where the bottlenecks occur and find ways to solve them. He said that in terms of imaging, a good way to think about it is using the Bayes’ theorem—the probability of an event, based on conditions that might be related to the event. How one makes a decision relating to a 49-year-old patient is very different to how one would make a decision relating to an 89-year-old patient; and a decision made one hour from symptom onset is very different to decision-making seven hours from symptom onset, he pointed out.
As it relates to the most efficient imaging, Goyal recommended multiphase computed tomography angiography (CTA) based collateral imaging, which was developed in the Calgary Stroke Program and was implemented in the ESCAPE trial and will also be used in ESCAPE 2 (ESCAPE NA1).
“I firmly believe that at two million neurons per minute, all that I am willing to spend on all the imaging, post-processing and decision-making is five minutes or 10 million neurons. As such, we do not do any magnetic resonance (MR) imaging or even CT perfusion imaging. In fact, we have found that decision-making based on head CT (ASPECTS score) and multiphase CTA is better than using CT perfusion for decision-making and of course takes significantly less time. The multiphase CT that we came up with is great for evaluating collaterals. It is 17 seconds of imaging time which can be done on any scanner, with no complex post-processing,” Goyal explained.

Comparing decision-making based on multiphase CT versus decision-making based on perfusion imaging, Goyal reported a case where CT showed good ASPECTS, proximal vessel occlusion, and good collaterals on multiphase CTA. The patient was taken to the cath lab, the vessel was opened and the patient returned to normal. On the other hand, he said, if perfusion imaging was used for decision-making in that case, based on current criteria, the team would have decided not to treat the vessel, because according to those criteria, the brain was already dead.
A key piece along the journey to refining the stroke workflow is the standardisation of technique. Goyal said this is something that they struggled with at Calgary, but which now runs smoothly according to the following factors: pre-notification; simple, decision-oriented imaging; parallel processing; and pre-organisation of the angiosuite.
In terms of pre-notification at Calgary, Goyal explained that this was set up with the paramedics so that before the patient arrives, the team already knows key simple information (age, last seen normal time, key deficit and expected time of arrival to the emergency room). Then, the stroke team is able to meet the patient at the entrance of the emergency room. Next, the team gathers for the multiphase CT angiogram where the decision-making is done. All members of the team converge at the CT scanner, imaging is viewed in real time, a decision is made and then the team splits up based on what each person needs to do. Throughout the process the team works in parallel where different parts of the team have their clear tasks and responsibilities. Each member takes care of what their role is without waiting for another part of the team to finish their part.
Once the decision is made to take the patient to the angiosuite, there is always a Brisk Recanalisation Ischaemic Stroke Kit already set up and ready, which, since there is hardly any time spent on opening packets and getting organised, Goyal said possibly saves over 30 minutes for cases done after working hours.
Finally, the one bottleneck that remains, according to Goyal, is getting the right patient to the right hospital as quickly as possible. In order to resolve this bottleneck, Goyal and colleagues are turning to mathematical modelling.
“We know that going to the wrong hospital takes a lot of additional time. Data from the HERMES collaboration show that if you go to the wrong hospital, it takes a phenomenal amount of time before you get to the correct hospital. There have been talks about setting up a drip and ship philosophy, but that is not the direction in which we are going. We are moving away from the idea of the hub and stoke model and we are doing mathematical modelling to try to come up with mathematics to decide how we make these decisions. The point of decision making is the first medical contact (usually a paramedic at the patient’s house). At this point, the key factors in the mathematical model are a) time to primary stroke centre b) time to comprehensive stroke centre c) expected door-in-door-out (DIDO) at the primary stroke centre d) time to transport from the primary stroke centre to the comprehensive stroke centre. Of course, there would be other factors like severity of stroke (likelihood of a proximal vessel occlusion) and other resource considerations. We have already built the first iteration of the model (currently under review at one of the leading journals),” he explained.
Summarising, Goyal maintained that stroke teams should always bear in mind that “speed is of the essence, the currency is neurons, the only thing that works is opening the vessel—the rest of them are just steps along the way, and everything is about making choices and teamwork. The only bottleneck that remains is getting the correct patient to the correct hospital fast, and that is what we have to collectively work on as we move forward.”













