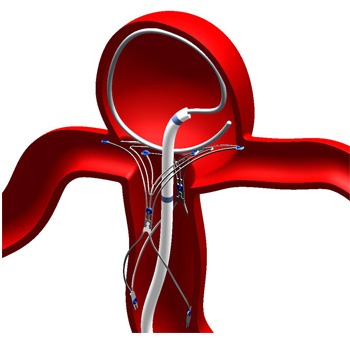
Codman Neuro, part of DePuy Synthes Companies of Johnson & Johnson, has announced that it has reached an exclusive distribution agreement with Pulsar Vascular to market and promote that company’s PulseRider in Europe, the Middle East and Africa. PulseRideris a minimally invasive device intended for use with embolic coils in the treatment of unruptured wide-neck intracranial aneurysms originating on or near a bifurcation. The device received initial CE mark approval in Europe in late 2013. The agreement was entered into by one of Codman Neuro’s EU affiliated companies.
The announcement was made at the European Society of Minimally Invasive Neurological Therapy (ESMINT) Congress. This distribution agreement marks the latest expansion of Codman Neuro’s neurovascular portfolio, which includes a wide range of products for haemorrhagic and ischaemic stroke, cerebral aneurysms and other neurovascular and neurological diseases and conditions. PulseRider is a self-expanding nitinol implant that is used in conjunction with embolic coils to bridge the neck of cerebral aneurysms.
“We are excited about this new agreement with Pulsar Vascular and the greater access physicians and their patients in Europe will now have to a potentially lifesaving endovascular procedure,” says P Laxmin Laxminarain, worldwide president of Codman Neuro. “There is a significant unmet clinical need in the treatment of unruptured, wide-neck bifurcation aneurysms, and we hope to help fill it with this innovative device.”
“Repairing wide-neck intracranial aneurysms is a challenging endovascular procedure and treatment options are extremely limited,” says Monika Killer-Oberpfalzer, Paracelsus Medical University Salzburg, Austria. “We welcome technology specifically designed to enhance our ability to treat these complex aneurysms more easily with less risk in the hope that more lives can be saved.”
PulseRider is limited to investigational use only in the United States and has not been approved by the US Food and Drug Administration (FDA) for distribution.













