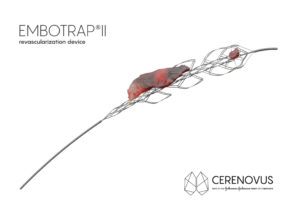
 Cerenovus has announced it has received 510(k) clearance from the US Food and Drug Administration (FDA) for EMBOTRAP II revascularisation device, a next generation stent retriever used to capture and remove life-threatening blood clots from the brain following an ischaemic stroke.
Cerenovus has announced it has received 510(k) clearance from the US Food and Drug Administration (FDA) for EMBOTRAP II revascularisation device, a next generation stent retriever used to capture and remove life-threatening blood clots from the brain following an ischaemic stroke.
The EMBOTRAP II device is designed to rapidly restore blood flow by gripping and retrieving clots within the neurovasculature, with minimal compression, protecting against further complications. The specific design of this mechanical thrombectomy device, which is indicated for use within eight hours of stroke symptom onset, is the result of extensive research into the science of clot.
In the ARISE II study, which was submitted as part of the 510(k) application to FDA, neurointerventional stroke physicians were able to restore blood flow in 80% of patients treated within three passes and in about half of patients within a single pass. At the 90-day follow-up, more than two-thirds were functionally independent. The study included 228 patients with large vessel occlusions and moderate to severe neurological deficits.
“Mechanical thrombectomy with newer generation devices is increasingly becoming standard treatment for acute ischaemic stroke,” said Osama Zaidat, Stroke and Neuroscience Medical Director of St. Vincent Mercy Hospital, Ohio, USA, and lead author of the ARISE II study, which was published in the peer-reviewed journal, Stroke, earlier this year. “The EMBOTRAP II Device with its high first pass rate and its ability to address such a broad range of clot is a crucial step forward for stroke treatment in America.”
The device is also approved for use in Europe, where more than 3,000 patients have been treated.













