urmila
RapidAI announces software approval for Medicare New Technology Add-on Payment
RapidAI states Rapid LVO is among the first software products to qualify for the New Technology Add-on Payment (NTAP).
NTAP is part of the CMS...
New ischaemic stroke guidelines widen mechanical thrombectomy window to 24 hours...
The American Heart Association and American Stroke Association released updated ischaemic stroke guidelines that were published in Stroke, and released during the International Stroke...
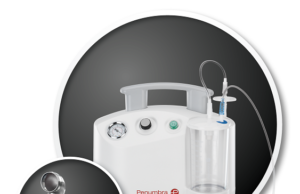
“Aspiration first” approach with Penumbra system demonstrates favourable outcomes for acute...
Penumbra announced results of the company-sponsored PROMISE study, demonstrating real-world safety and efficacy of the Penumbra system with ACE 68 and ACE64 reperfusion catheters...
Medtronic gets US FDA clearance for Riptide aspiration system
The Riptide aspiration system is intended for use in the revascularisation of patients with acute ischaemic stroke secondary to intracranial large vessel occlusive disease...
Endovantage gains US FDA 510(k) clearance for Surgicalpreview
Endovantage has announced receiving 510(k) clearance from the US FDA for Surgicalpreview, its preoperative planning system for the endovascular treatment of cerebral aneurysms.
Precise sizing...