By Andreas MJ Frölich
Endovascular aneurysm therapy today is characterised by a rapidly growing and evolving collection of available treatment devices. The challenge in achieving optimal results lies not only in the manual skill of an individual operator, but certainly also in selecting the best treatment approach for a given patient’s aneurysm.

Both planning and performing these operations requires an intimate understanding of the way endovascular devices work and behave in patient anatomy. Our research group has focused on the application of 3D-printed, patient-specific neurovascular models to perform endovascular treatments in vitro. With this approach, we hope to find new ways for operators to learn and practice endovascular interventions. Our goal is to provide a framework in which operators can learn the basics of endovascular treatment, maintain and improve their skills with devices that they use less commonly, as well as familiarise themselves with entirely new therapeutic devices.
To create a neurovascular model, we typically start out with 3D rotational angiography image data. Although it is possible to use CT or MR data as the source, the higher spatial resolution offered by 3D rotational angiography drastically improves the model’s accuracy. We then manually segment out the aneurysm and adjacent vasculature. Artifacts need to be removed and non-essential vascular branches must be shortened or clipped in order to integrate the models into a flow circuit. After segmentation, connectors for silicone tubes need to be added digitally and the image data must be converted to a format suitable for 3D printing. Among the plethora of different manufacturing techniques, fused deposition modelling, stereolithography and material jetting currently appear most relevant for neurovascular modelling. We manufacture only some of our models ourselves with on-site printers. External manufacturers have helped us gain access to some of the more advanced (and more expensive) additive manufacturing techniques, including material jetting. This now allows us to obtain highly precise, elastic neurovascular models that excellently replicate the imaging anatomy (Figure).
We utilise the models as part of training courses for physicians learning or already practicing endovascular aneurysm therapy. One of the greatest advantages of working in a patient-specific anatomy is that course participants have the ability to perform endovascular procedures in vitro after studying the same case in an interactive workshop format. We first review the clinical case by studying real clinical angiography images and fluoroscopy videos. The interventional strategy as well as any actual clinical problems and possible solutions are then discussed and participants can formulate their own treatment plan and discuss alternatives. In the experimental angiography suite, participants then have the opportunity to perform the procedure themselves, encountering almost identical anatomy inside the models. The previously devised different treatment strategies can be compared, such as the influence of microcatheter shape on gaining aneurysm access or the effects of choosing different coil sizes and degrees of stiffness. In repeat instances, we have now observed that clinical challenges that had occurred in patients, such as difficulties with aneurysm access, were replicated in the 3D printed models and could be overcome by the same solutions that worked clinically.
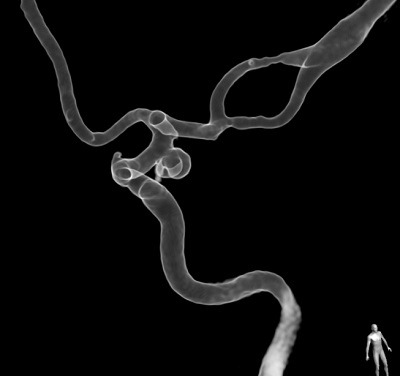
In my opinion, the ability to work in a patient-specific environment opens up exciting possibilities to improve medical education. Being able to retrospectively select clinical cases that were challenging or associated with complications will allow the definition of standardised training curricula that could make sure that interventionalists are familiar with common peri-interventional problems and their solutions, at least those problems that are directly related to a patient’s anatomy. Further improvement in the current design of neurovascular models is certainly necessary. For example, endoluminal friction and vessel wall elasticity differ from reality due to the employed materials. Important complications such as thrombosis and vasospasm are also currently not replicated in vitro. However, using the elastic models with relatively thin walls, the possibility for peri-interventional aneurysm rupture can be integrated into the models, increasing the degree of realism.
In commercial aviation, before the widespread use of simulation for training aircrew, pilots were trained by undergoing a sequence of increasingly difficult exercises on real aircraft1. The widespread acceptance of simulators for training and certification of aircrew has been attributed to a combination of technological advances in simulation and the development of standards for flight simulation, which led to greater acceptance among pilots and regulatory authorities. In the field of neuroradiology, technological advances in additive manufacturing now greatly enhance the scope and versatility of model-based neurointerventional training. It is my hope that these technical developments will be accompanied by a more widespread adaptation of in vitro endovascular training and thus ultimately contribute to improved patient safety in neuroradiology.
Reference(s)
- Page RL. Brief history of flight simulation. SimTecT 2000 proceedings. 2000:1-11. doi: 10.1.1.132.5428.
Andreas MJ Frölich is at the Department of Diagnostic and Interventional Neuroradiology, University Medical Center Hamburg-Eppendorf, Hamburg, Germany













