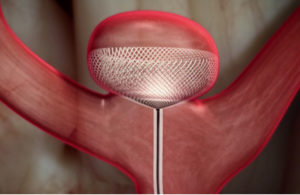
 The Contour Neurovascular System (Cerus Endovascular) will offer a new option for treating intracranial aneurysms. According to Kyriakos Lobotesis, Principle Clinical Advisor at Cerus, “the objective of the device is to treat the neck of the aneurysm, thus avoiding protracted manoeuvres and manipulation within the aneurysm, which can be experienced while using other available devices”.
The Contour Neurovascular System (Cerus Endovascular) will offer a new option for treating intracranial aneurysms. According to Kyriakos Lobotesis, Principle Clinical Advisor at Cerus, “the objective of the device is to treat the neck of the aneurysm, thus avoiding protracted manoeuvres and manipulation within the aneurysm, which can be experienced while using other available devices”.
Can you describe the Contour Neurovascular System?
The Contour Neurovascular System is an innovative, intrasaccular, self-anchoring, embolisation device targeting the aneurysm neck.
How is it different from existing devices for the treatment of intracranial aneurysms, such as coils, intrasaccular flow disruptors and flow diverters?
The device, due to its unique placement across the neck of the aneurysm, acts as both a flow disrupter and diverter. Unlike other intrasaccular devices the Contour Neurovascular System is not as limited by aneurysm size or shape. Its unique stabilising design architecture prevents it from migrating or compacting into an aneurysm post-deployment. The device mesh provides a uniform scaffold distributed across the neck of the aneurysm for the establishment of neointimal development and unlike devices placed in the parent vessel, is not dependent on the use of dual antiplatelet therapy. Hence it can be used for the treatment of both ruptured and unruptured intracranial aneurysms.
How does the device work and what are the objectives?
The objective of the device is to treat the neck of the aneurysm, thus avoiding protracted manoeuvres and manipulation within the aneurysm, which can be experienced while using other available devices. The aim is to offer consistent neck coverage and stability to reduce the risk of compaction/recanalisation/retreatment. The device works by a combination of diversion and disruption of flow in the aneurysm sac.
What were the results of the animal studies?
Over a number of animal studies, the device has consistently demonstrated high rates of complete occlusion of the aneurysm, which were sustained over a period of time. Thrombosis and neointimal growth across the neck were repeatedly confirmed histopathologically. Deliverability, positioning and device stability were also excellent throughout. These results, and other testing performed, provided the confidence to move forward into first human use.
What types of intracranial aneurysms can the Contour device be used to treat?
Because the Contour System addresses the neck of the aneurysm, it is not limited to specific aneurysm location or morphology, therefore it can be used to treat a wide range of intracranial aneurysms. Its ease of deliverability and accurate positioning allow it to address even difficult to treat wide-necked bifurcation aneurysms and aneurysms with branches arising from their side wall. As mentioned above, being intrasaccular, its use does not necessitate dual antiplatelet therapy. It hence can be used for the treatment of both ruptured and unruptured intracranial aneurysms.
What were the results of the first-in-man study?
First-in-man studies have confirmed device stability at six months and steady aneurysm occlusion during this period. The patients all maintained their pre-treatment status and most importantly there were no adverse events. The patients will of course continue to be followed-up.
What is planned in terms of future trials?
The first trial has been approved and is already recruiting patients. A second multicentre trial is currently seeking approval and will start enrolling patients shortly.













